LymphoSign : CE/IVD kit for the classification of non-Hodgkin's lymphomas
Non-Hodgkin’s lymphoma is a type of cancer that originates in the lymphatic system, which is part of the immune system. It is characterized by the abnormal growth of lymphocytes, a type of white blood cell, and can occur in any part of the body.
There are more than 80 types of non-Hodgkin’s lymphoma, some of which are rare and difficult to characterize. Accurate knowledge has become a key issue.
Many advances have been made since the introduction of the WHO classification in 2001. However, the risk of errors remains higher than in other pathologies.
The LymphoSign test offers a detailed classification of these lymphomas. It is a state-of-the-art method for evaluating the stage of differentiation in non-Hodgkin’s lymphoma (NHL) tumor cells. By leveraging RNA expression levels of over 130 relevant gene markers, this solution provides valuable insights into disease progression.
Non-Hodgkin’s Lymphoma Subtypes
High Grade B-cell Non-Hodgkin’s Lymphoma
- DLBCL ABC
- DLBCL GCB
- DLBCL PMBL
Low Grade B-cell Non-Hodgkin’s Lymphoma
- Follicular Lymphoma (FL)
- Mantle Cell Lymphoma (MCL)
- Marginal Zone Lymphoma (MZL)
- Small Lymphocytic Lymphoma (SLL)
T-Cell Non-Hodgkin’s Lymphoma
- Angioimmunoblastic T-cell Lymphoma (AITL)
- ALK-positive Anaplastic Large Cell Lymphoma (ALCL ALK+)
- ALK-negative Anaplastic Large Cell Cytotoxic Lymphoma (ALCL ALK- cytotox)
- ALK-negative Anaplastic Large Cell Th2 Lymphoma (ALCL ALK- Th2)
- Adult T-cell Leukemia-Lymphoma (ATLL)
- NK/T-cell Lymphoma (NKTCL)
Test Characteristics
LymphoSign is an innovative, accurate, and reliable test for pathologists and molecular biologists. This test is used to characterize non-Hodgkin lymphomas (NHL).
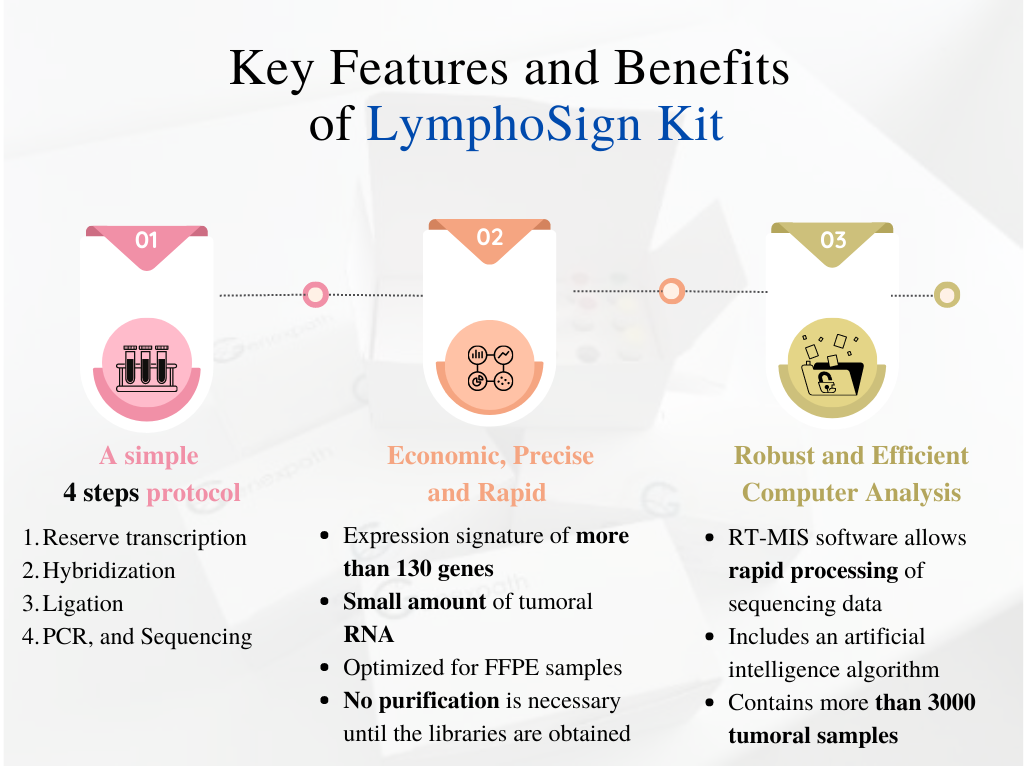
It assesses the degree of differentiation of tumor cells by analyzing the expression level of more than 130 relevant genetic markers, based on ligation-dependent PCR technology (RT-MLPSeq).
The simplicity of the test allows results to be obtained within 24 hours from tumor RNA. The protocol is optimized for low-quality samples such as FFPE samples.
Using artificial intelligence trained on a database of more than 3,000 cases, the RT-MIS platform establishes the most likely classification among 13 subtypes of B-cell and T-cell NHL.
What is RT-MLPSeq?Key Protocol Features
Sample Preparation
- No purification required until library preparation, minimizing material loss and maintaining high sensitivity.
- Short genetic sequences targeted (40-60 bases), ensuring robustness against RNA degradation.
- Particularly suitable for analyzing difficult biological samples like paraffin-fixed tissue biopsies.
Sequencing
- 10⁵ sequences per sample sufficient for analyzable expression profiles, allowing high-throughput analysis.
- Libraries can be loaded simultaneously with other sequencing libraries, optimizing cost efficiency.
Analysis
- Once sequencing is completed, the FASTQ file can be uploaded to the RT-MIS platform.
- After a few minutes of analysis, a report with the expression signature of various genes and an AI-predicted classification according to the 2017 WHO classification is provided.
Technical Specifications
| Handling Duration | ≃4 hours |
|---|---|
| Actual Working Time | ≃1 to 1.5 hours |
| Type of Nucleic Acid | RNA |
| Input Quantity | Between 50 and 500 ng of RNA in a volume of 2 µl |
| Type of Cancer | Non-Hodgkin Lymphoma |
| Contents of the Reagent Kit | Probes targeting 137 markers, barcodes, sequence primers |
| Method | Ligation-dependent RT-PCR |
| Description | Compare the expression profiles obtained from genes, somatic mutations, or chromosomal translocations with those of the main types of non-Hodgkin’s lymphoma. |
| Equipment Compatibility | MiSeq, NextSeq 500, NextSeq 550 Illumina® |
| Type of Samples | Tissue biopsies at room temperature, frozen, or fixed and included in paraffin |
| Technology | Next Generation Sequencing |
Key Features and Benefits
Available Kits
Resources
Publications
-
High PDL1/PDL2 gene expression correlates with worse outcome in primary mediastinal large B-cell lymphoma
Vincent Camus et al., Blood Advances, December 12, 2023
Read the article -
Integrative diagnosis of primary cutaneous large B-cell lymphomas supports the relevance of cell of origin profiling
Audrey Gros et al., PLoS One, April 22, 2022
Read the article -
Circulating tumor DNA in primary mediastinal large B-cell lymphoma versus classical Hodgkin lymphoma
Vincent Camus et al., Leukemia & Lymphoma, April 2022
Read the article -
Defining signatures of peripheral T-cell lymphoma with a targeted 20-marker gene expression profiling assay
Fanny Drieux et al., Haematologica, June 2020
Read the article -
Combining gene expression profiling and machine learning to diagnose B-cell non-Hodgkin lymphoma
Victor Bobée et al., Blood Cancer Journal, May 22, 2020
Read the article -
LymphoSign Test-Kit
Download the Publication
Training Video
Medical Device Notice
These products are medical devices.
Please follow the manufacturer’s instructions.


