GRP94 Antibody
Katalog-Nummer OASE00006
Size : 200ug
Marke : Aviva Systems Biology
| Datasheets/Manuals | Printable datasheet for GRP94 Antibody (OASE00006) |
|---|---|
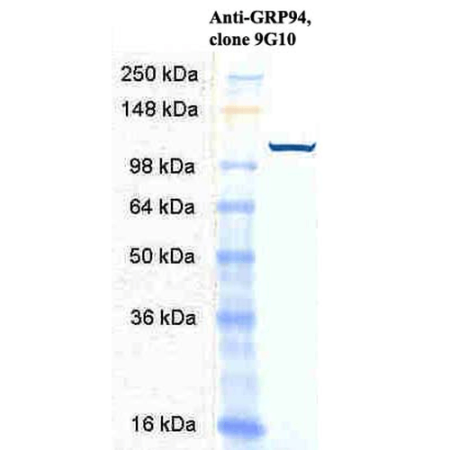
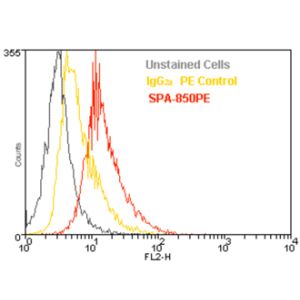
| COA Datasheet | 0.5 µg/ml of SMC-105 was sufficient for detection of Grp94 in 20 µg of heat shocked HeLa cell lysate by colorimetric immunoblot analysis using Goat anti-rat IgG:HRP as the secondary antibody. |
| Predicted Species Reactivity | Bovine|Chicken|Dog|Guinea Pig|Hamster|Horse|Human|Monkey|Mouse|Porcine|Rabbit|Rat|Sheep|Xenopus|Xenopus laevis |
|---|---|
| Clonality | Monoclonal |
| Clone | 9G10 |
| Isotype | IgG2a |
| Host | Rat |
| Conjugation | Unconjugated |
| Application | FC,ICC,IF,IP,WB |
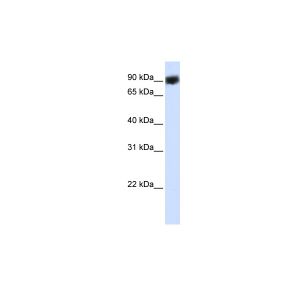
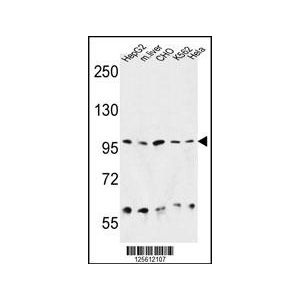
| Additional Information | Background Info: Detects a 98kDa protein corresponding to the molecular mass of Grp94 on SDS PAGE immunoblots. Does not detect human Hsp90, Grp74, or GrpE from E.coli. |
| :: | Scientific Background: Grp94 (glucose regulated protein 94, gp96) is a constitutively expressed endoplasmic reticulum (ER) lumenal protein that is up-regulated in response to cellular stress such as heat shock, oxidative stress or glucose depletion. Grp94 is thought to play a role in protein translocation to the ER, in their subsequent folding and assembly, and in regulating protein secretion (1). Grp94 also plays a role in antigen presentation by accessing the endogenous pathway and eliciting specific CTL responses to chaperone bound peptides via MHC class I pathway (2) Grp94 is a member of the Hsp90 family of stress proteins and shares sequence homology with its cytosolic equivalent, Hsp90 (3). Both Hsp90 and Grp94 are calcium binding proteins (4). Despite sharing 50% sequence homology over its N domains and complete conservation in its ligand binding domains with Hsp90, Grp94 and Hsp90 differ in their interactions with regulatory ligands as Grp94 has weak ATP binding and hydrolysis activity (5). Grp94 exists as a homodimer and the two subunits interact at two distinct intermolecular sites, C terminal dimerization domains and the N-terminal interacts with the middle domain of opposing subunits. (6). Grp94 contains a carboxy terminal KDEL (Lys-Asp-Glu-Leu) sequence which is believed to aid in its retention in the ER (7). |
| :: | Certificate of Analysis: 0.5 ug/mL was sufficient for detection of Grp94 in 20ug of heat shocked HeLa cell lysate by colorimetric immunoblot analysis using Goat anti-rat IgG:HRP as the secondary antibody. |
| Reconstitution and Storage | -20°C |
| Immunogen | Purified Grp94 isolated from chicken oviducts |
| Purification | Protein G Purified |
| Concentration | 1 mg/ml |
| Specificity | Detects ~98kDa. Does not detect human HSP90, Grp74, or GrpE from E.coli. |
| Dilution | WB (1:2000), ICC/IF (1:100); optimal dilutions for assays should be determined by the user. |
| Storage Buffer | PBS pH7.2, 50% glycerol, 0.09% sodium azide |
| Description | Grp94 (glucose regulated protein 94, gp96) is a constitutively expressed endoplasmic reticulum (ER) lumenal protein that is up-regulated in response to cellular stress such as heat shock, oxidative stress or glucose depletion. Grp94 is thought to play a role in protein translocation to the ER, in their subsequent folding and assembly, and in regulating protein secretion (1). Grp94 also plays a role in antigen presentation by accessing the endogenous pathway and eliciting specific CTL responses to chaperone bound peptides via MHC class I pathway (2). Grp94 is a member of the HSP90 family of stress proteins and shares sequence homology with its cytosolic equivalent, HSP90 (3). Both HSP90 and Grp94 are calcium binding proteins (4). Despite sharing 50% sequence homology over its N domains and complete conservation in its ligand binding domains with HSP90, Grp94 and HSP90 differ in their interactions with regulatory ligands as Grp94 has weak ATP binding and hydrolysis activity (5). Grp94 exists as a homodimer and the two subunits interact at two distinct intermolecular sites, C terminal dimerization domains and the N-terminal interacts with the middle domain of opposing subunits (6). Grp94 contains a carboxy terminal KDEL (Lys-Asp-Glu-Leu) sequence which is believed to aid in its retention in the ER (7). |
| Reference | 1. Rudolph R.W., and Bedows E. (1997) J Biol Chem 272: 3125-3128. 2. Srivastava P.K., et al. (1994) Immunogenetics. 39(2):93- 98. 3. Mazzarella R.A., and Green M. (1987) J Biol Chem 262: 8875-8883. 4. Kang, H.S. and Welch W.J. (1991) J Biol Chem 266(9): 5643-5649. 5. Soldano K.L., et al. (2003) J Biol Chem 278(48): 48330- 48338. 6. Chu F., et al. (2006) Protein Sci 15(6): 1260-1269. 7. Peter F., et al., (1992) J Biol Chem 267: 10631-10637. 8. Allen S. et al. (2000) Blood 96(2): 560-568. 9. Sato K et al. (2001) Blood 98(6): 1852-1857. 10. Yun S.-W. et al (2000) Brain Research Bulletin.52(5): 371-378. 11. Choukhi A., et al. (1998) J. Virol. 72: 3851-3858. 12. Hoshino T., et al. (1998) Blood 91(11): 4379-4386. 13. Riera M. et al. (1999) Mol. Cell Biochem. 191: 97-104. 14. Gusarova V., et al. (2001) J. Biol. Chem. 276(27): 24891- 24900. |
|---|---|
| Gene Symbol | HSP90B1 |
| Gene Full Name | heat shock protein 90 beta family member 1 |
| Alias Symbols | 94 kDa glucose-regulated protein, ECGP, endoplasmin, endothelial cell (HBMEC) glycoprotein, epididymis luminal protein 35, epididymis secretory sperm binding protein Li 125m, GP96, gp96 homolog, GRP94, heat shock protein 90 kDa beta member 1, heat shock protein 90kDa beta (Grp94), member 1, heat shock protein 90kDa beta family member 1, HEL-S-125m, HEL35, stress-inducible tumor rejection antigen gp96, TRA1, tumor rejection antigen (gp96) 1, tumor rejection antigen 1. |
| NCBI Gene Id | 7184 |
| Protein Name | Endoplasmin|endoplasmin precursor [Homo sapiens] |
| Description of Target | Molecular chaperone that functions in the processing and transport of secreted proteins. When associated with CNPY3, required for proper folding of Toll-like receptors (By similarity). Functions in endoplasmic reticulum associated degradation (ERAD). Has ATPase activity. |
| Uniprot ID | P14625 |
| Protein Accession # | https://www.ncbi.nlm.nih.gov/protein/NP_003290.1 |